The Pluristyx Platform
Accelerating Stem Cells from Concept to Clinic.
The Pluristyx Platform is developing the next generation of stem cell therapies. Currently it is slow, hard, and expensive to develop cell therapies. Pluristyx has the expertise needed to take on the most difficult parts of your therapeutic development, including off-the-shelf and custom induced Pluripotent Stem Cells (iPSCs), process and analytical development services, gene editing, and cell line development, to help convert your concept into a clinical reality.
The Pluristyx Platform
A novel platform for rapid, efficient and high-quality iPSC therapy development. Our platform comprises a comprehensive suite of iPSCs developed from our proprietary cellular reprogramming technology. This end-to-end iPSC solution allows groups to test drive off-the-shelf lines including those incorporating our proprietary gene edits FailSafe® and iACT Stealth Cells™. Each pillar of the platform is designed to address key development milestones.
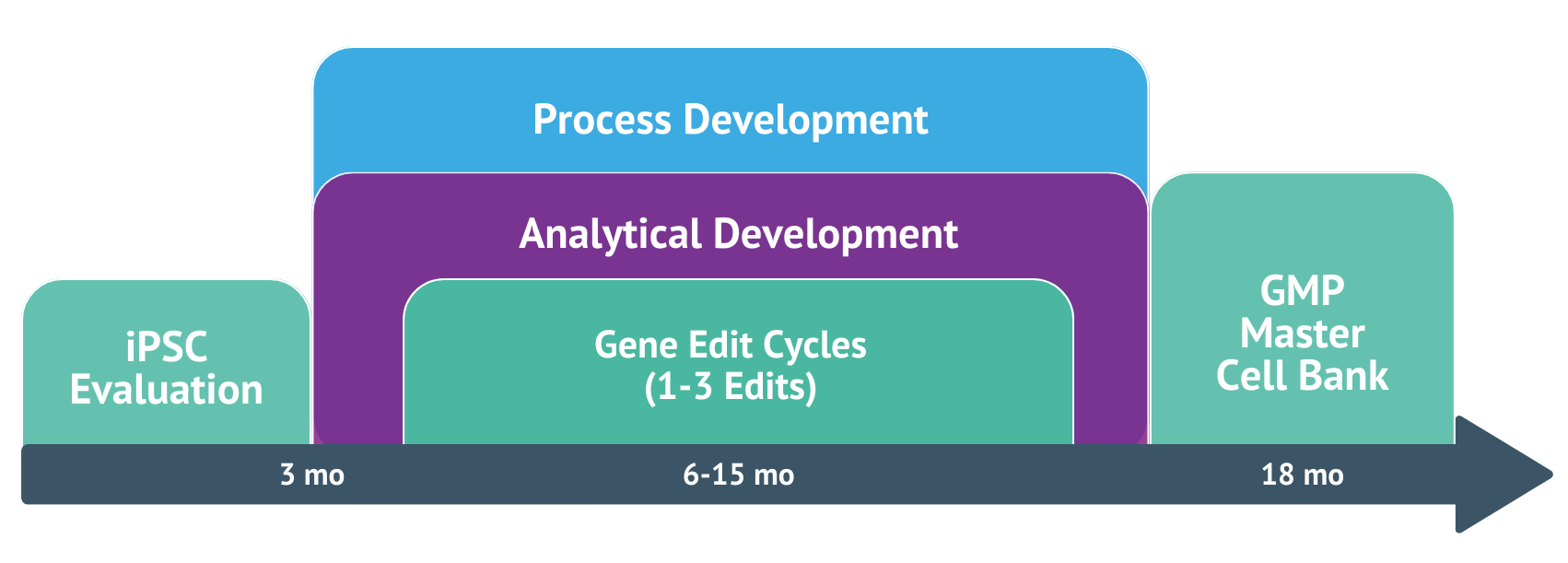
Using over 50 years of executive experience in development and manufacturing, The Pluristyx Platform is a full-service, product, and technology platform that expedites your path from proof of concept to commercialization. With The Pluristyx Platform, you can obtain GMP edited iPSC banks within 18 months- complete with all necessary quality and regulatory documentation.
Our clients have seen a 70% reduction in development timelines and ultimately lower downstream costs. The Pluristyx Platform provides a single point of access for proprietary technologies with improved freedom to operate (FTO) compared to alternative solutions. Pluristyx's comprehensive suite is provided by Pluristyx’s Seattle based site.
The Pluristyx Platform
A novel platform for rapid, efficient and high-quality iPSC therapy development. Our Platform comprises a comprehensive suite of iPSCs developed from our proprietary cellular reprogramming technology. This end-to-end iPSC solution allows groups to test drive off-the-shelf lines including those incorporating our proprietary gene edits FailSafe® and iACT Stealth Cells™. Each pillar of the platform is designed to address key development milestones.
Using over 50 years of executive experience in development and manufacturing, The Pluristyx Platform is a full-service, product, and technology platform that expedites your path from proof of concept to commercialization. With The Pluristyx Platform, you can obtain GMP edited iPSC banks within 18 months- complete with all necessary quality and regulatory documentation.
Our clients have seen a 70% reduction in development timelines and ultimately lower downstream costs. The Pluristyx Platform provides a single point of access for proprietary technologies with improved freedom to operate (FTO) compared to alternative solutions. Pluristyx's comprehensive suite is provided by Pluristyx’s Seattle site.
The Pluristyx Platform Development Timeline
70% reduction in development time
Proprietary technology with clear freedom to operate
Fastest path to clinic
Case Studies
Whether you are looking for proprietary cell lines and banks, manufacturing and quality consumables, gene editing technologies, or development and GMP manufacturing services, Pluristyx gives partners the opportunity to engage us at any step in the process. The Pluristyx Platform was designed to address and overcome challenges in cell therapy development to make, Tomorrow's Cell Therapies Today®.
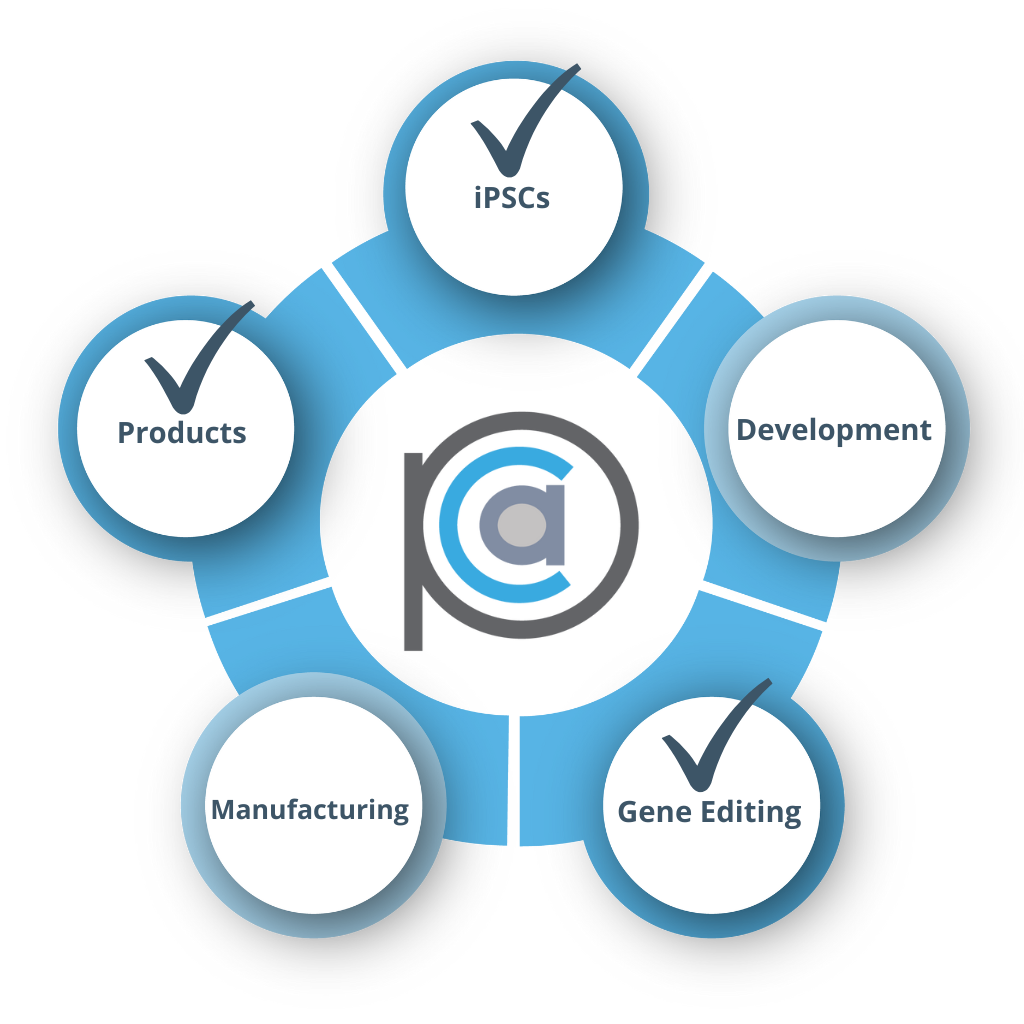
Large-Cap Pharm
- Internal GMP capabilities
- Seeking new iPSC lines and allogeneic cell technologies
- RESULT: Commercial license executed for iPSC lines with FailSafe® & iACT Stealth Cells™
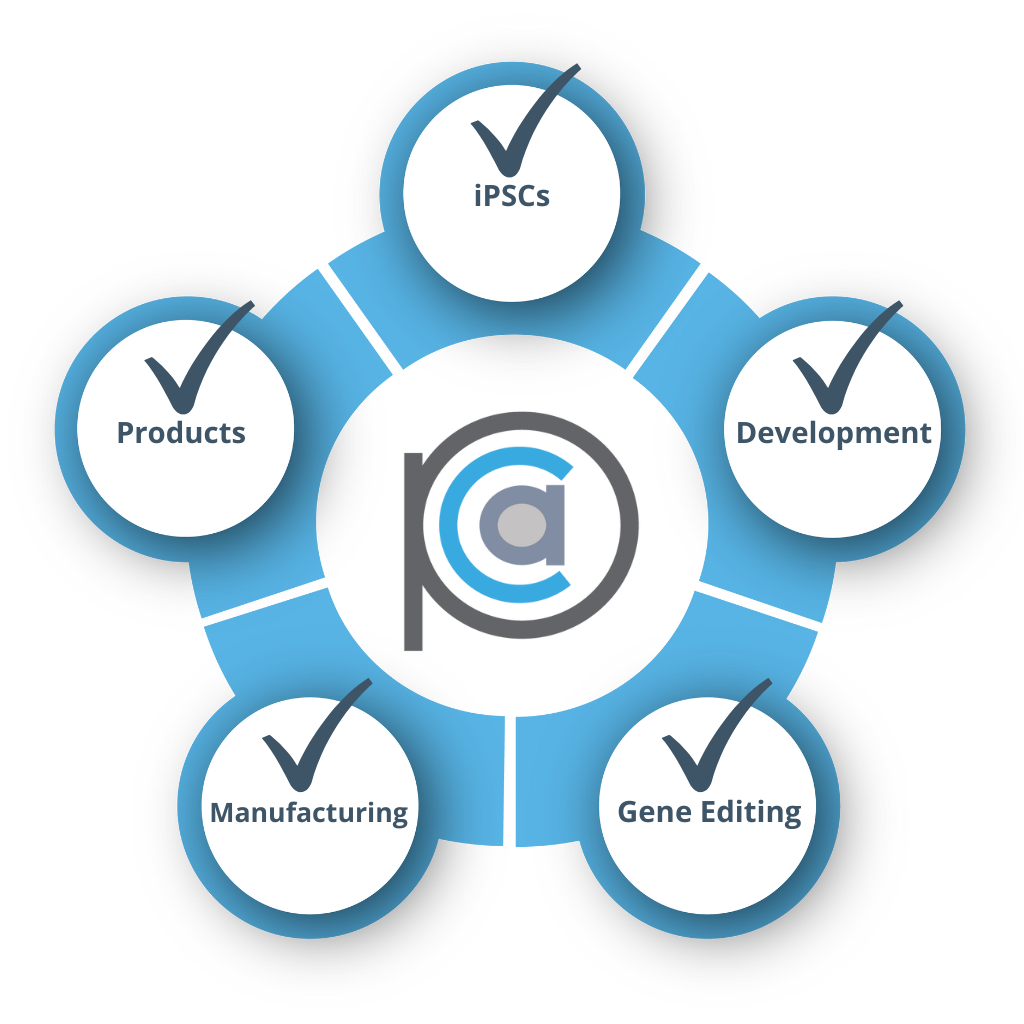
Non-Profit
- Partnering with leading technology providers
- Looking for an end-to-end partner with holistic capabilities
- RESULT: Engagement with full panCELLa Platform
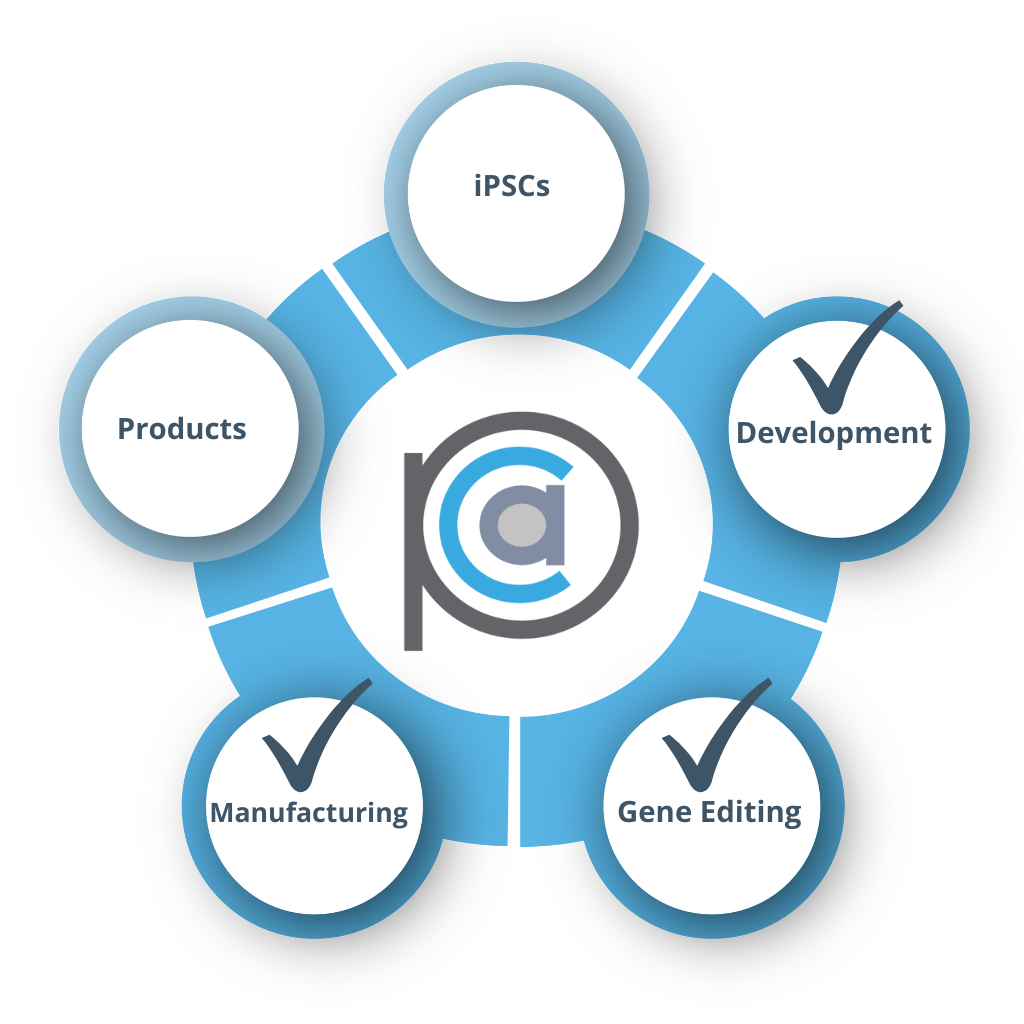
Mid-size US Biotech
- Already purchased cell lines
- Seeking GMP capabilities, gene editing and process development expertise
- RESULT: GMP process designed and executed in 12 months
Large-Cap Pharm
- Internal GMP capabilities
- Seeking new iPSC lines and allogeneic cell technologies
- RESULT: Commercial license executed for iPSC lines with FailSafe® & iACT Stealth Cells™

Non-Profit
- Partnering with leading technology providers
- Looking for an end-to-end partner with holistic capabilities
- RESULT: Engagement with full panCELLa Platform

Mid-size US Biotech
- Already purchased cell lines
- Seeking GMP capabilities, gene editing and process development expertise
- RESULT: GMP process designed and executed in 12 months

Download Brochure

