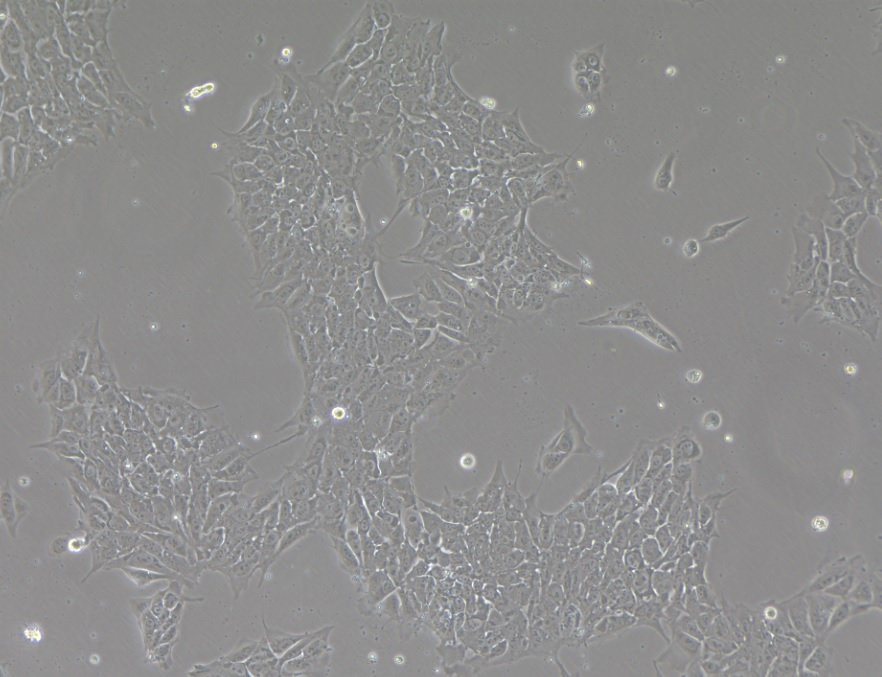
Description
PSXi001 Human induced Pluripotent Stem Cells
Catalog Number: PSXi001-A
Cell number per vial: 1 M and 25 M
Product Summary
PSXi001 Human Induced Pluripotent Stem Cells (hiPSCs) were derived from the dermis of juvenile foreskin procured under current Good Tissue Practice (cGTP) and conform to Global Ethical Standards and Clinical Cell Regulations. PSXi001 hiPSCs are intended for Research Use Only (RUO) and shall not be developed for diagnostic or therapeutic use. Pluristyx PSXi001 cells are free of mycoplasma, pathogens, bacteria, yeast, and fungus, and guaranteed to achieve >80% viability post-thaw at the indicated cell number. PSXi001 cells were characterized for karyotype, pluripotency, and Short Tandem Repeat (STR) cell line identity.
PSXi001 are available in different formats: standard bank vial (> 1 M cells), and Ready to Differentiate (RTD™) high density vials of pluripotent stem cells at 25 million cells/vial suitable for thaw and immediate use.
Format: Research Use Only; not for diagnostic or therapeutic purposes
Passage number: <25
Karyotype: 46, XY
Pluripotency Markers: OCT4 & SSEA-4 (>90%); SSEA-1 (<10%)
These cells are for Research Use Only (RUO) and shall not be developed for diagnostic, therapeutic, or commercial use.