Top Biotech Canadian Company by Life Sciences Review
Accelerating the Development of
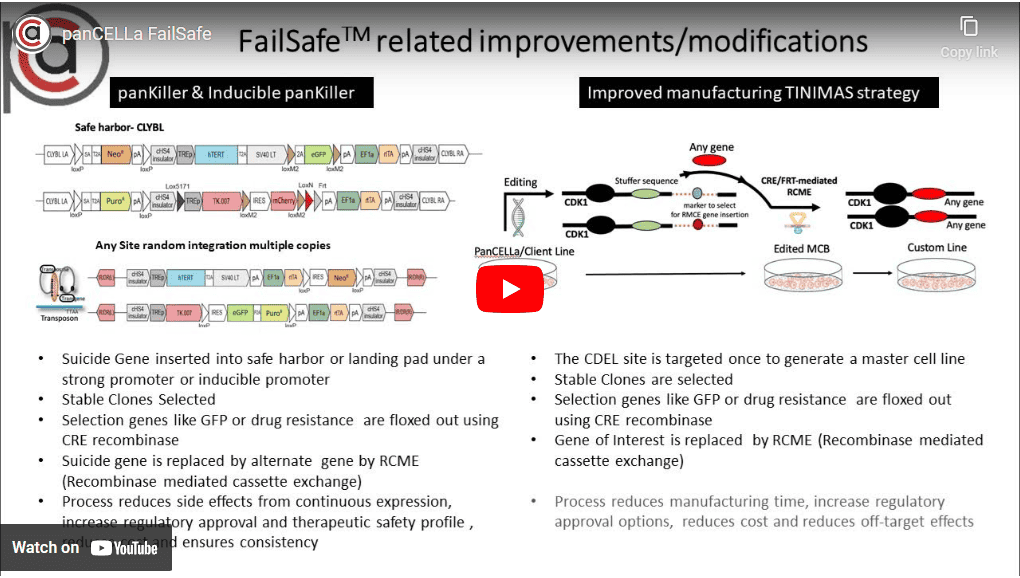
Advanced Cell Therapies | PanCELLa is a pioneering company in the development of pluripotent stem cells and gene editing technology for creating “off-theshelf” cell therapies.
Top Biotech Canadian Company by Life Sciences Review Read More »